Health and a healthy environment
Human health and promotion of health for all through a healthy environment is a central objective. WHO has defined healthy People 2020 Environmental Health objectives >>> and health and a healthy environment are an important element of the UN -sustainable developmental goals. >>> Still a main problem of health is unequal access to health-relevant resources and social aspects.
Health, nature preservation, conversion of land use >>> intensification of agriculture and environmentally sustainable food production are broadly discussed being in a problematic tension and need improved governance. >>>
Consumers and markets
Consumers are the most important group and NGO in the regulation of markets and consumers’ objectives are therefore of central importance. >>>
Objectives such as health, safety, informed decision, variety of choices, environmental health, sustainable food production, and organic farming are typically commonly agreed objectives. BEUC >>> However, different cultural backgrounds and education need to be respected in often considerably divergent objectives >>> and choices of ways of health protection.
The group of well-informed consumers mostly desire detailed information including possible advantages as well as risks being able to draw personal conclusions which may be different from recommendations of authorities. Strict authority regulations may then be opposed as paternalism. The “nanny state” discussion has a principal political dimension. >>> Other consumers may not be interested in details of products and authorities are made responsible for their safety. For example biohacking or DIY biology, scientifically often seen as unsafe and biased, gain attraction. >>>
Regulations
“There are widespread inconsistencies and contradictions in the many published definitions of ‘nutraceuticals’ and ‘functional foods’, demonstrating uncertainty about what they actually are. There are no internationally agreed definitions of ‘nutraceuticals’ and ‘functional foods’, or of similar terms, such as ‘health foods’, or of terms related to herbal products, which are sometimes referred to as ‘nutraceuticals’. The term ‘dietary supplement’ is widely used to designate formulations that are also called ‘nutraceuticals’, ‘Fortified foods’, sometimes called ‘designer foods’, are foods to which compounds of proven therapeutic or preventive efficacy (e.g. folic acid) have been added. (Aronson, 2017) (Santini et al., 2018).
In the US, recent legislation regarding food safety includes the Food Safety Modernization Act, signed in 2011 by Barack Obama. FSMA has given the Food and Drug Administration (FDA) new authorities to regulate the way foods are grown, harvested, and processed. The FSMA requires the FDA to undertake more than a dozen rule makings and issue at least 10 guidance documents, as well as a host of reports, plans, strategies, standards, notices, and other tasks. FDA’s guidance documents cover e.g. Food facility registration, Current Good Manufacturing Practices (CGMPs), Hazard Analysis & Critical Control Points (HACCP), Retail Food Protection, Imports & Exports. >>> , >>> US- FDA regulates food for special medical purposes. >>>
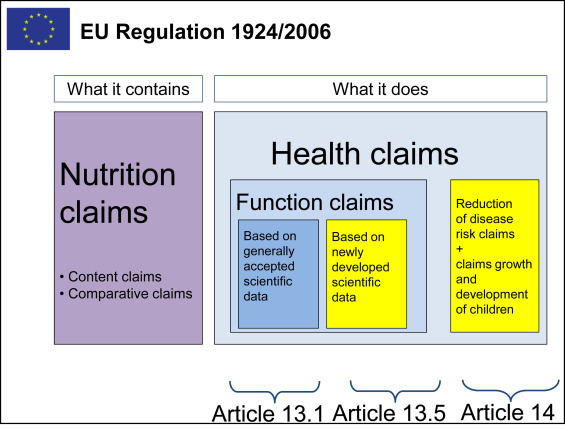
The European Union has implemented a regulatory system for marketing and labeling of foods a new regulation came into force on 1st of January 2018 replacing previous regulations. >>>
Special regulations apply for supplements, dietary supplements, medicinal foods, botanicals. Allocation of products to the categories is often difficult and scientific benchmarks to achieve standards are often vague. The European panel on food additives and flavorings evaluates safety. >>>
EC 1924/2006 on nutrition and health claims made on foods” defines health claims as “any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health”. The two principal categories of health claims are reduction of disease risk claims and claims referring to children’s development and health (Article 14) claims other than those referring to the reduction of disease risk and children’s development and health (Article 13).
General functions of health claims. >>> ; >>> , Opinions of the EFSA panel on the substantiation of health claims in the case of quercetin. >>>
In the tension between science and scientific uncertainties, health and precautionary aspects, consumer information and consumer objectives, environmental sustainability, industrialised agriculture as well as useful technological developments and market interests this regulation and panel opinions became into fierce discussion and critics. >>>
Whereas regulations are of central importance unclear regulations may result in monopolisation of markets where only big players can handle these regulations resulting in hindrance of the development of improved products >>>
The use and value of scientific citations in the evaluation of health functions of e.g. of plant ingredients are vague and recommendations for successful substantiation of new health claims are under controversial discussion >>>
International harmonisation in these areas seems to be of crucial importance to achieve internationally agreed goals of safe foods and
.