Aging, healthy aging, epigenetic, longevity
Aging mechanisms
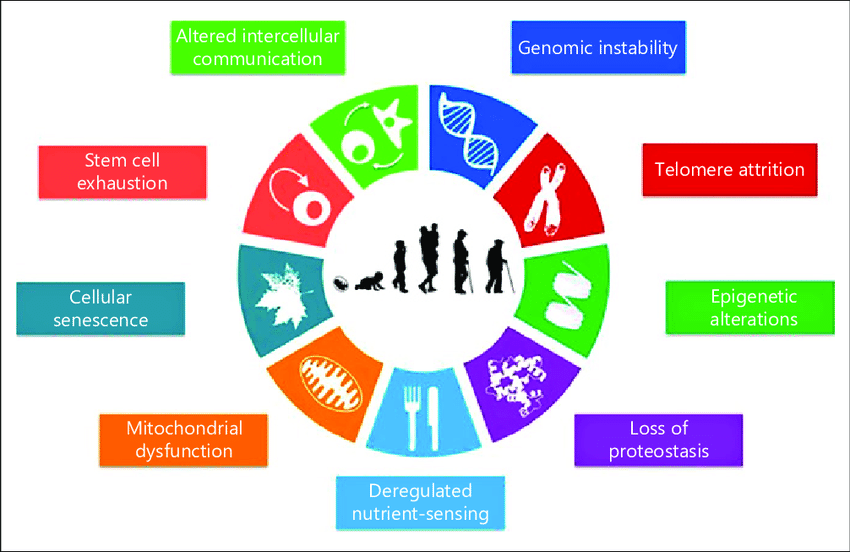
Aging is a complex process accompanied by loss of physiological integrity and higher mortality. This deterioration leads to higher risk for non-communicable diseases like cancer, cardiovascular diseases, neurodegenerative diseases and diabetes.
At the current state of science there are nine main hallmarks of aging described.
All of this aging-relevant factors can be positively influenced by an adequate diet and lifestyle
Epigenetic markers & the biological clock
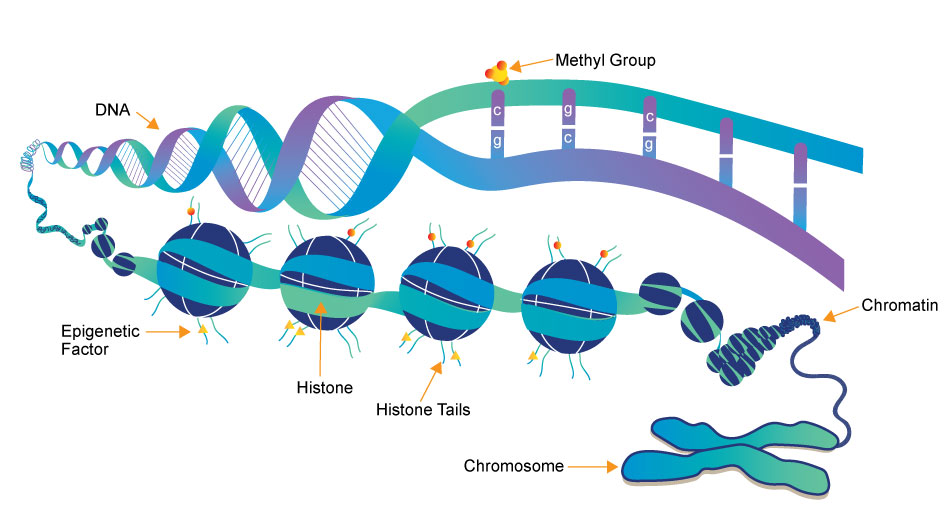
Epigenetic regulation is the sequence independent regulation ot the DNA by signals such as from nutrition, lifestyle or social stress.
Methylation of DNA is a part of epigenetics. When cytosines in regulatory regions of a gene become methylated by methyk transferases transcription of these genes is usually reduced.
Global DNA methylation and aging
A great number of CpGs methylation is analysed. With advancing age, generally a decrease in global DNA methylation is observed.
Site-specific DNA methylation and aging
In contrast to global DNA methylation, some GpGs show an increase in methylation with higher age. The smaller the amount of CpGs analysed, the more their specific relations to aging fall into amount.ck
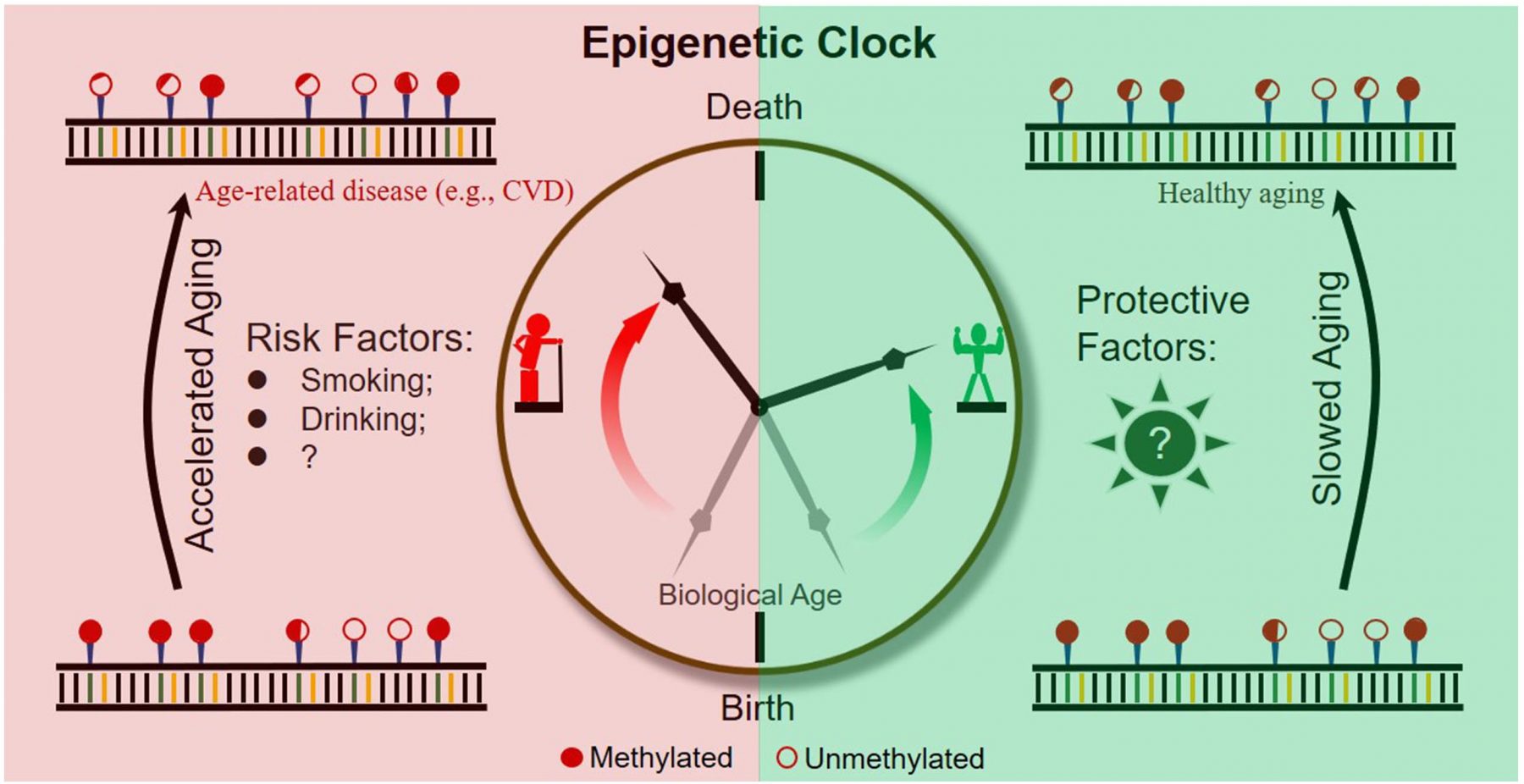
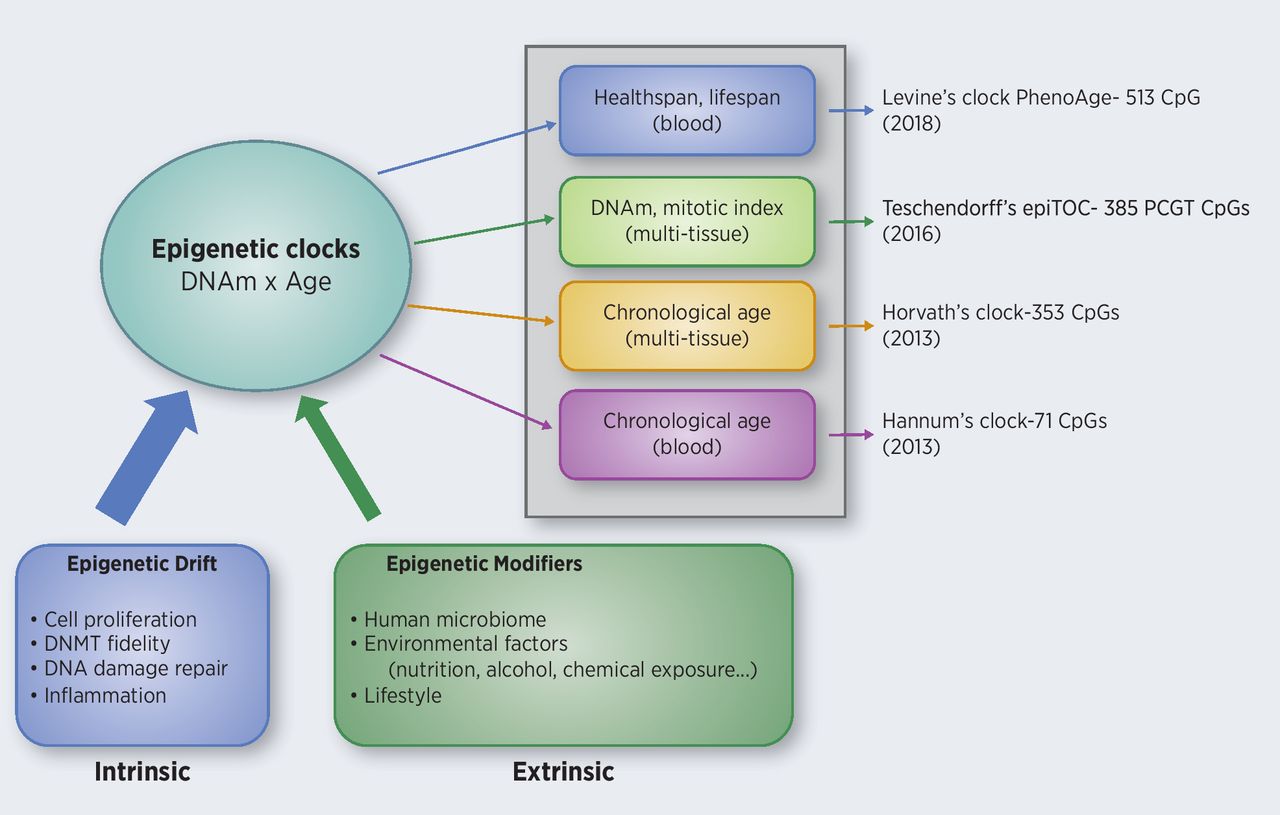
The methylation of specific age-related CpGs can predict age very accurately. In 2013 Horvath successfully devised an multi tissue age predictor for age with a correlation of 0,96 to chronologicage and an error margin of 3,6 years. The 353 observed CpGs have the same methylation patterns with ongoing age, independent of tissue. This means that the Epigenetic Clock ticks the same in slow proliferating brain cells and in a fast proliferating blood cells.
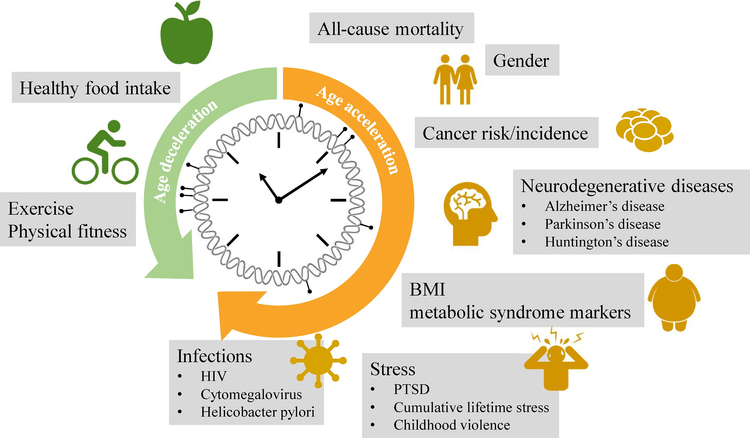
Why are people older than their chronological age?
There are several reasons for increased epigenetic aging. Lifestyle and nutrition are aspects which can make people younger or older. Obesity is correlated with an increase in age. Factors like intake of fish, fruit, vegetable as well as education and sport can lead to lesser signs of ageing. This means that an adequate intake of nutrients, can prevent from premature aging and help to stay healthy. Other reasons can be viral infections, neurological deceases, cancer and hormonal imbalance.
Epigenetic age and mortality
The epigenetic age correlates with the mortality risk. An age acceleration of 5 years means a 16 % increase in mortality risk.
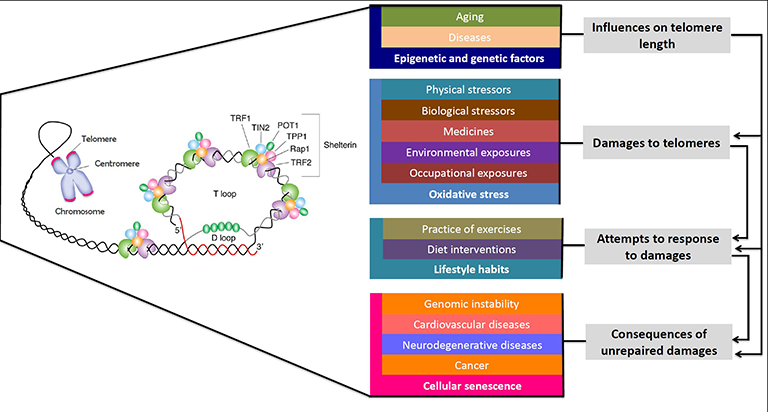
Telomeres
Chromosomes contain the genetic information of an organism are built up of DNA-molecules. Telomeres consist of repetitive 5′-TTAGGG-3′-sequences and are located at the ends of chromosomes. This specialized structures, out of DNA and Proteins, offer protection and stability. They can be compared with the Ends of shoelaces. Extremely shortened telomeres can lead to instability of the genome and in consequence to diseases.
With every cell division telomeres are shortened, until DNA cannot be replicated anymore. This leads to the state of senescence (no more cell division) or to programmed cell death (apoptosis). This means that the division of normal body cells is limited.
Senescent cells (SC) contribute to the aging process and age-related diseases due to pro-inflammatory signalling which can affect the whole organism.
Many studies have shown, that humans with good plant-based nutrition and an active lifestyle have longer telomeres than control groups. Phytochemicals like polyphenols (f.i. from green tea) can also help to keep telomeres longer.
Telomere length, telomerase activity is tightly regulated by epigenetic mechanisms involving h TERT
Apoptosis, autophagy and senescence
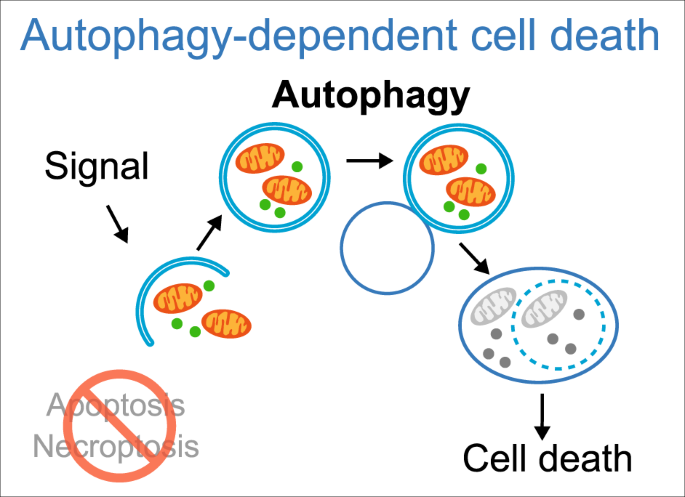
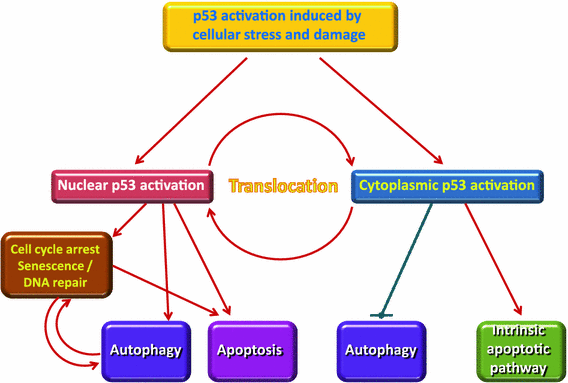
Autophagy is a cleanup process that causes the removal of aggregated or misfolded proteins an also the recycling of damaged cell components, plays a big role in this. Autophagy is up-regulated by fasting and CR and has a protective part against genome instability and necrosis. By and large, it has a substantial role in prevention of many diseases like infections, diabetes, cancer, autoimmune diseases and others,
Apoptosis is an orderly process in which the cell’s contents are packaged into small packets of membrane for “garbage collection” by immune cells. Apoptosis removes cells during development, eliminates potentially cancerous and virus-infected cells, and maintains balance in the body.
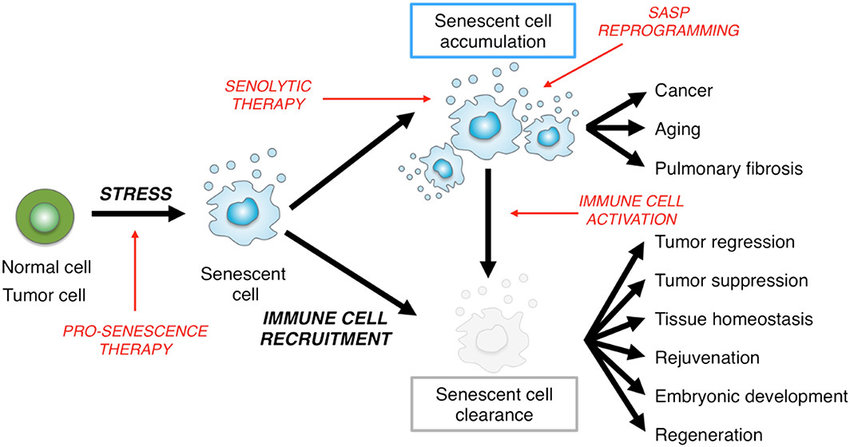
Cellular senescence is one phenomenon by which normal cells cease to divide. In the 1960’s, Leonard Hayflick and Paul Moorhead found out that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. Senescence can be induced by such stresses as telomere shortening, DNA damage, oncogenic mutations, metabolic and mitochondrial dysfunction, and inflammation. Senescent cell burden increases in multiple tissues with aging, at sites of pathology in multiple chronic diseases, and after radiation or chemotherapy.
Senescent cells (SC) play a big role in the process of aging and for age-related physical dysfunctions. SC are very different from healthy cells. The expression of different genes, no more replication and the increased production of pro-inflammatory signals are characteristics. Such signaling substances are f.i. inflammatory promoting cytokines, chemokines, and built together the so called senescence-associated secretory phenotype (SASP). The inflammation created by SC can lead to tissue dysfunction and as well can turn healthy cells senescent.
Senescence cells have several pathways to escape apoptosis. This is where senolytics come into play. Xu and colleagues found out, that a mix of dasatinib and quercetin (flavonoid) selectively eliminate SC and so alleviated physical dysfunction an late-life survival in mice. Now a number of pharmaceuticals and function foods/ additives and especially caloric restriction / fasting are investigated for their effects to reduce senescence cells from tissues. This would have a deep impact on longevity, health with economic aspects.
Metabolic pathways in aging, fasting, IGF, insulin signalling and SIRT
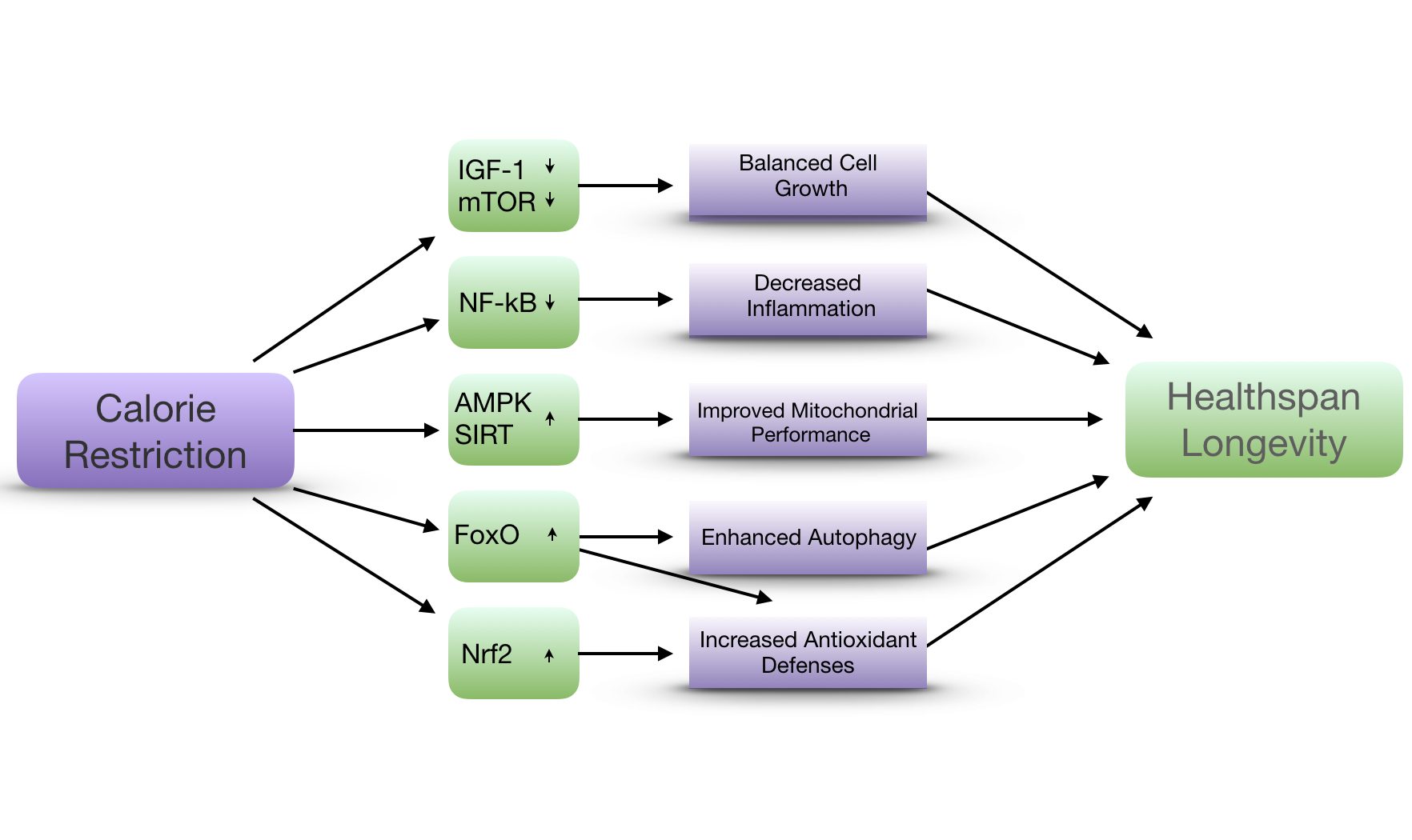
When food is ingested, growth hormones (GH) are released and activate the insulin an IGF-1 signalling pathway to provide cells with information about the glucose status, allowing them to react accordingly. A constant activation of this metabolic pathway can be harmful to the organism. Mammals with a genetic polymorphism that reduces GH and IGF-1 signalling have shown to have increased lifespan and a better ability to resist stress conditions.
Fasting leads to a reduction of anabolic pathways and induces catabolic signalling (e.g. AMPK, FOXO and sirtuins). For instance, SIRT 1 shows positive effects by triggering the metabolic switch and turning endogenous fatty acids into the main source of energy. AMPK is activated by sensing low levels of AMP and blocks mTOR which leads to stimulation of autophagy and interaction with SIRT1 to a positive feedback loop.molecules.stem cell functions
Whereas high level of ROS (radical oxygen species) accelerate aging, low levels of ROS seem to be necessary to continue the state of quiescence and the self-renewal ability. Fasting can decrease ROS through ketogenic metabolism.